Intraperitoneal chemotherapy as first-line treatment of ovarian cancer
Radosław Mądry, Janina Markowska, Jolanta Lubin
 Affiliacja i adres do korespondencji
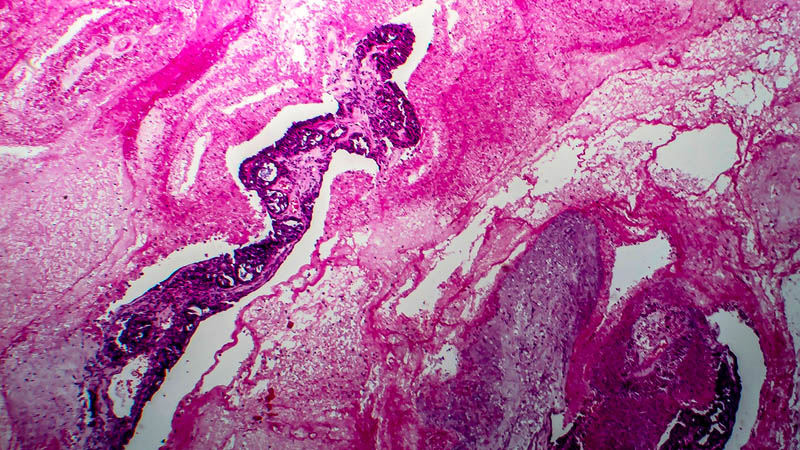
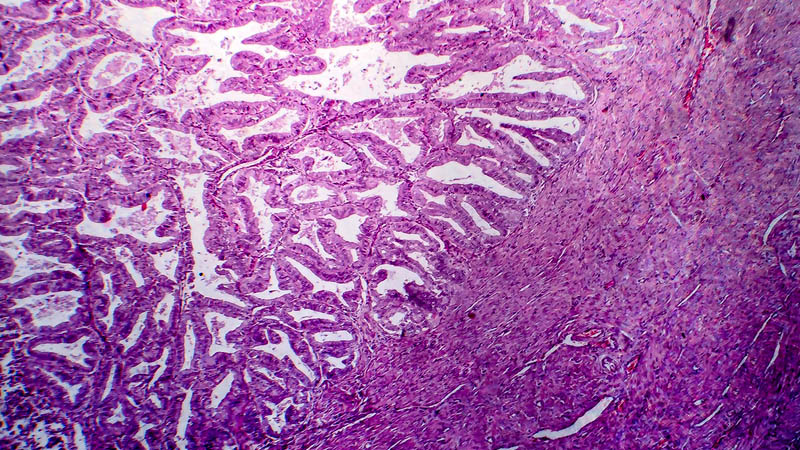
Affiliacja i adres do korespondencjiIntraperitoneal chemotherapy (IP) in the treatment of ovarian cancer became lately an attractive alternative to standard intravenous chemotherapy. This was a consequence of publication in 2006 of phase III trials indicating that this approach provided clinical benefit as compared with traditional approach in terms of progression-free survival (24 vs. 18 months) and overall survival (66 vs. 50 months, respectively). The present paper reviews theoretical basis of IP, recapitulates milestones of research leading to introduction of IP as first-line therapeutic option and discusses IP-associated adverse effects. Discussion includes impact of IP on patients’ quality of life and effectiveness of other protocols, e.g. those replacing cisplatin by carboplatin. The authors highlight several problems associated with a more widespread use of IP in the treatment of ovarian cancer and indicate novel trends in research, heralded by publications concerning once-weekly administration of paclitaxel. At present, IP as first-line therapy is indicated in FIGO stages II-IV, with residual disease of less than 1 cm after primary cytoreduction. Side effects associated with this therapeutic modality may be classified as drug-dependent and catheter-dependent. The may be minimized by proper selection of patients and port-care. At present, several clinical trials are underway, testing other dosage regimens and chemotherapy protocols. Concomitantly, clinical trials being performed, enable inclusion of patients after IP chemotherapy.